The Interplay of COVID-19, Gut Health, and Immunity: New Insights
Written on
Chapter 1: Understanding Gut Dysbiosis and COVID-19
Gut dysbiosis is associated with adverse outcomes in COVID-19 and can hinder the clearance of the virus, often persisting even after recovery.
COVID-19 can lead to gastrointestinal symptoms, including nausea and diarrhea, in approximately 10% of cases. Any disruption to gut health inevitably impacts the gut microbiota, as the composition of these microbes is closely linked to the host's gut physiology. For instance, gut inflammation can create a "leaky gut," disrupting the balance of microbial populations. A deficiency in butyrate-producing bacteria can result in insufficient butyrate levels, which are crucial for healing the gut lining and reducing inflammation.
The pivotal question remains: Does COVID-19 induce significant changes in gut microbiota, or does pre-existing gut dysbiosis exacerbate the severity of COVID-19? Gaining clarity on this matter is crucial, as the gut microbiota is known to influence multiple other organs in the body.
Section 1.1: The Research Behind Gut Microbiota Changes
A study published in Gastroenterology by a team from the Chinese University of Hong Kong analyzed the gut microbiome of 15 patients with moderate to severe COVID-19, alongside six pneumonia patients and 15 healthy individuals. They monitored changes in gut microbiota over an average hospital stay of 21 days, relating these alterations to various disease metrics.
This video discusses how COVID-19 impacts gut health and can lead to long-term symptoms, as explained by a pharmacist.
The findings revealed that COVID-19 patients exhibited a higher prevalence of opportunistic pathogens, such as Clostridium hathewayi, Actinomyces viscous, and Bacteroides nordii, compared to control groups. Notably, these changes were consistent regardless of age, sex, pre-existing conditions, or antibiotic use. Additionally, beneficial gut microbes like Fecalibacterium prausnitzii and Lachnospiraceae bacterium were significantly reduced in COVID-19 patients.
Worse gut microbiota disturbances were observed in patients who received antibiotics during their treatment. Even ten days post-recovery, 10 out of 15 former COVID-19 patients continued to exhibit gut dysbiosis, with their microbial profiles remaining significantly altered compared to individuals who had never contracted the virus.
Section 1.2: Predicting COVID-19 Severity Through Gut Microbiota
The researchers further investigated whether the gut microbiota present upon hospital admission could predict the severity of COVID-19 in patients. They identified three bacterial species—Coprobacillus, Clostridium ramosum, and Clostridium hathewayi—that were strongly linked to increased disease severity. Conversely, Alistipes onderdonkii and Fecalibacterium prausnitzii were associated with better outcomes, highlighting their roles in gut health and inflammation reduction.
Subsection 1.2.1: The Role of Gut Microbiota in Viral Clearance
The study pinpointed 14 gut bacteria associated with effective viral clearance, particularly noting that some Bacteroidetes species might protect against SARS-CoV-2 by reducing ACE2 expression in the gut. In contrast, the presence of Erysipelotrichaceae species correlated with poor viral clearance and more severe COVID-19 outcomes.
To Sum Up the Study
It's important to note that this study was based on a small sample of 15 moderate to severe COVID-19 patients, so its findings may not extend to milder cases. However, it revealed several groundbreaking insights:
- COVID-19 patients displayed a distinct gut bacterial profile marked by the depletion of beneficial species and an increase in opportunistic pathogens compared to pneumonia and healthy controls.
- Gut dysbiosis persisted in the majority of patients even after recovery and clearance of the virus.
- Certain baseline gut microbiota could predict the likelihood of experiencing severe COVID-19.
- Specific Bacteroidetes species were linked to more effective viral clearance.
- Patients receiving antibiotics showed the most severe gut dysbiosis.
Chapter 2: The Gut-Lung Axis and Therapeutic Implications
This video features Dr. Ken Cadwell and Dr. Jonas Schluter discussing how COVID-19 affects gut health and its implications for recovery.
Numerous studies have begun exploring the gut-lung axis in relation to COVID-19, investigating whether dietary or probiotic interventions could have therapeutic benefits. The gut-lung axis is believed to operate bidirectionally, where inflammation in the lungs can impact gut microbiota and vice versa.
Research has indicated that a diet rich in nutrients that support gut microbiota could enhance lung immunity. Additionally, studies on mice have shown that antibiotic-induced gut dysbiosis may heighten susceptibility to respiratory viruses, with germ-free mice displaying increased mortality from lung infections.
Would Probiotics Help?
In humans, meta-analyses of randomized controlled trials suggest that probiotics might reduce the incidence and duration of respiratory infections. Some studies have indicated improved outcomes in pneumonia patients receiving probiotics compared to those on placebo. However, the authors caution against the indiscriminate use of conventional probiotics for COVID-19 until more direct evidence is available.
Despite this, some researchers advocate for the use of probiotics, asserting their safety even among vulnerable populations. They suggest that further investigation into probiotics' role during the COVID-19 pandemic is warranted.
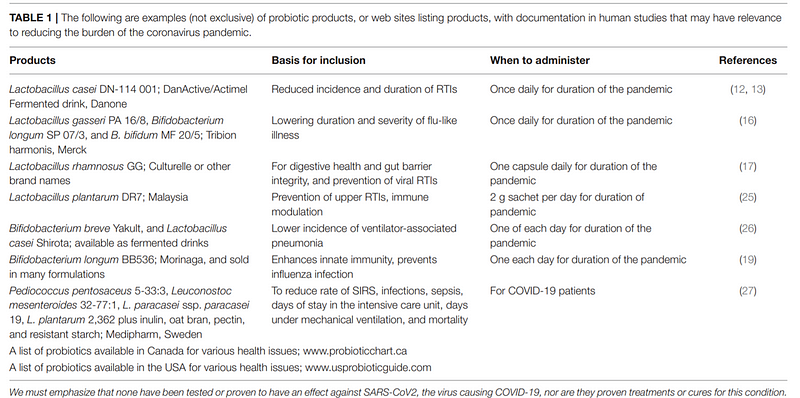
Open-access source (CC BY): Baud D, Dimopoulou Agri V, Gibson GR, Reid G and Giannoni E (2020) Using Probiotics to Flatten the Curve of Coronavirus Disease COVID-2019 Pandemic. Front. Public Health 8:186. doi: 10.3389/fpubh.2020.00186